Continuously refrigerated from manufacture to delivery at your office.
You’re probably unaware of the far-reaching effects of refrigeration of whitening gels, and the lack thereof. Storage and shipping temperatures affect far more than you might think.
Whitening gels break down during storage and shipping if not refrigerated
 Heat is the enemy of whitening gel potency1…and gel potency is a big key to ensuring totally satisfied whitening patients.
Heat is the enemy of whitening gel potency1…and gel potency is a big key to ensuring totally satisfied whitening patients.
All peroxide based whitening gels are unstable chemicals – they’re supposed to be.2-6 This is why they’re able to break down quickly in the mouth, releasing bleaching factor byproducts. The downside of this chemical instability is that all whitening gels start degenerating immediately after manufacture if not kept under constant refrigeration.4,5,7-9
The end product of any peroxide based whitening gel (including carbamide peroxide) is hydrogen peroxide (HP). HP may break down in two different pathways. Depending on which pathway, HP may break down to:
- Water and molecular oxygen, or…
- Hydroxyl radicals, perhydroxyl radicals, superoxide radicals, oxygen ions and hydrogen ions (acid)
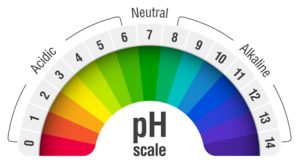
Water and molecular oxygen do virtually nothing to whiten teeth. We need the breakdown pathway #2 above because it is the radicals (especially perhydroxyl radicals) that are mostly responsible for whitening.19-28 But notice that in addition to releasing effective radicals, hydrogen ions (acid) are also created, which lowers the pH of whitening gels.
 When whitening gels break down prematurely, not only do they lose potency/effectiveness, but they also become far more acidic.
When whitening gels break down prematurely, not only do they lose potency/effectiveness, but they also become far more acidic.
Even storing whitening gels at room temperature (73.4°F) can double and triple acid concentration of the gels.8 The higher the temperature, the greater the degradation process.7,8 Warm, or even hot, temperatures that may be encountered during warehouse storage at chemical manufacturers and whitening product companies, is problematic. Even more problematic is the extreme temperature (125°F – 165°F) typically encountered in freight truck bays during truck shipment10 of gels from chemical factories to whitening companies, and from whitening companies to your office. Just think of how hot your closed car gets on a sunny day!11 This extreme heat often causes severe heat degradation of the whitening gels, which means you receive whitening gels that have lost potency, and sometimes have lost most of their potency.4,5,7-9 Is it any wonder that you experience such a wide range of effectiveness with typical whitening systems?

As NON-refrigerated whitening products break down and degrade during storage and shipping, hydrogen ions are produced.8,12 Those hydrogen ions are acid (pH = potential of Hydrogen). Whitening gels therefore become more and more acidic as they break down, resulting in an ever-increasing possibility of sensitivity and pain.4
Constant refrigeration virtually stops breakdown of whitening gels.
Refrigeration fully protects whitening gels against the potency-robbing damage of heat, and even room temperature, during storage and shipping.8
At-home whitening gels stored at room temperature (73.4°F) average more than double (120%) the acidity than when kept refrigerated (39°F).8
 In-office whitening gels (much higher concentration) stored at room temperature average more than triple (210%) the acidity than when kept refrigerated.8
In-office whitening gels (much higher concentration) stored at room temperature average more than triple (210%) the acidity than when kept refrigerated.8
The potency breakdown and more than doubling and tripling in acidity shown above is very significant.8 And that is only at room temperature. So just imagine when storage at the chemical factory and whitening product company is in a warmer warehouse. But far worse, the extreme temperatures of 125°F – 165°F during freight truck shipping10 from chemical factory to whitening company, and from whitening company to dental office has far more degradation impact.4
In addition to less potency/effectiveness of whitening gels when not refrigerated, the multiples of acidity may cause demineralization of tooth structure and loss of natural smear plugs resulting in opening of dentinal tubules and increased sensitivity.
But wait – there’s something else that happens: The increase in acidity/lowering of pH results in increased osmolality/hypertonicity.5,13-16 As stated in Brännström’s Theory of Dentinal Sensitivity, greater osmolality increases the osmotic “pull” on dentinal tubular fluid, significantly increasing sensitivity.
And one more thing: The lower (more acidic) the pH, the more the HP breaks down to only water and molecular oxygen. The higher (more basic) the pH, the more the HP breakdown is pushed toward formation of the effective radicals.1,17,18
As shown above, refrigeration prevents premature breakdown of whitening gels and release of hydrogen ions, which prevents the multiples of increase in acidity. This means higher pH, and more production of effective radicals instead of ineffective water and molecular oxygen.
The Bottom Line
 KöR Whitening is the first company in the world to constantly refrigerate a full line of teeth whitening products from the moment of manufacture, until you receive them cold-packed at your dental practice.
KöR Whitening is the first company in the world to constantly refrigerate a full line of teeth whitening products from the moment of manufacture, until you receive them cold-packed at your dental practice.
The result? You receive KöR Whitening gels at virtually the same potency and neutral pH as the day they were manufactured.1,4,12 You get exceptionally effective gels with much higher pH and lower osmolality (lower sensitivity) with less risk of pain for your patients.1,4,9,12 And with higher concentrations of effective radicals, when KöR gels are placed onto the warm teeth, they therefore break down with extraordinary effectiveness.

To see all the very detailed science and references relating to whitening sensitivity click on the The Science of Whitening Gel Refrigeration Science Paper below.
Get the full story on the science behind continuously refrigerating whitening gels…
References
- Andrea Freire, Lucí Regina Panka Archegas, Evelise Machado de Souza, Sérgio Vieira. Effect Of Storage Temperature On Ph Of In-Office And At-Home Dental Bleaching Agents. Acta Odontol Latinoam. 2009;22(1):27-31.
- Baratieri L N, Andrada M A C, Monteiro Jr. S, Vieira. L.C.C.P.Clareamento dental. São Paulo: Santos Editora, 2004. p.129.
- Ansel H C, Popovich N G, Allen Jr. L V. Farmacotécnica: Formas Farmacêuticas e Sistemas de Liberação de Fármacos. 6.ed. São Paulo: Premier, 2000. p.135-140.
- Bonesi C M, Ulian L S, Balem P, Angeli V W. Carbamide peroxide gel stability under different temperature conditions: is manipulated formulation an option? Braz. J. Pharm. Sci. vol.47 no.4 São Paulo Oct./Dec. 2011.
- Margeas RC. New advances in tooth whitening and dental cleaning technology. The Academy of Dental Therapeutics and Stomatology Dental Continuing Education Peer-Reviewed Web site. Accessed 2009;March.
- Greenwall, L. Bleaching Techniques in Restorative Dentistry. Martin Dunitz. London: 2001.
- Christensen G, Tooth Bleaching, State-of-Art ’97. Clinical Research Associates Newsletter 1997;21(4).
- Freire A, Archegas LR, de Souza EM, Vieira S. Effect of storage temperature on pH of in-office and at-home dental bleaching agents. Acta Odontol Latinoam. 2009;22(1):27-31.
- Chang, R. Quimica. Lisboa (Port): McGraw-Hill; 1994.
- United Parcel Service of America. Direct correspondence. 2008.
- Protect your car from the damaging effects of sun and heat. State Farm Insurance. https://www.statefarm.com/simple-insights/auto-and-vehicles/protect-your-car-from-the-damaging-effects-of-sun-and-heat. Accessed 2022 Dec.
- Eary LE. Catalytic decomposition of hydrogen peroxide by ferric ion in dilute sulfuric acid solutions Metallurgical and Materials Transactions B. 1985 June; 16(2):181-186.
- Abd-Elmeguid A, Yu DC. Dental Pulp Neurophysiology: Part 1. Clinical and Diagnostic Implications. JADC. 2009;75(1):55.
- Anderson DJ, Matthews B, Shelton LE. Variations in the sensitivity to osmotic stimulation of human dentine. Arch Oral Biol. 1967; 12(1):43–7.
- Narhi M, Kontturi-Narhi V, Hirvonen T, Ngassapa D. Neurophysiological mechanisms of dentin hypersensitivity. Proc Finn Dent Soc. 1992; 88 Suppl 1:15–22.
- Narhi MV, Hirvonen T. The response of dog intradental nerves to hypertonic solutions of CaCl2 and NaCl, and other stimuli, applied to exposed dentine. Arch Oral Biol. 1987;32(11):781–6.
- Sun G. The role of lasers in cosmetic dentistry. Dent Clin North Am 2000;44:831-850.
- Howe-Grant M, editor. Encyclopedia of chemical technology, 4th ed., vol. 13. New York: John Wiley and Sons; 1992.
- Joiner A. The bleaching of teeth: A review of the literature. Journal of Dentistry. 2006; 34(7).
- Delfino CS, ChinelattiII MA, Carrasco-GuerisoliI LD, Batistalil AR. Effectiveness of home bleaching agents in discolored teeth and influence on enamel microhardness. Journal of Applied Oral Science. 2009; 17(4).
- McCaslin AJ, Haywood VB, Potter BJ, Dickinson GL, Russell CM. Assessing dentin color changes from nightguard vital bleaching. Journal of the American Dental Association. 1999; 130.
- Joiner A, Thakker G. Evaluation of a 6% hydrogen peroxide tooth whitening gel on enamel and dentine microhardness in vitro. Journal of Dentistry. 2004; 32(Suppl. 1).
- White DJ, Kozak KM, Zoladz JR, Duschner HJ, Gotz H. Effects of tooth-whitening gels on enamel and dentin ultrastructure—a confocal laser scanning microscopy pilot study. Compendium of Continuing Education in Dentistry. 2000; 21(Suppl. 29).
- Sulieman M, Addy M, Macdonald E, Rees JS. The bleaching depth of a 35% hydrogen peroxide based in-office product: a study in vitro. Journal of Dentistry. 2005; 33.
- Goldberg M, Bohin F, Bonnet E, Claisse-Crinquette A, Dartigues J, Louis J. Tooth Bleaching Treatments: A Review. Association Dentaire Française, Paris. 2005.
- Heymann HO. Tooth whitening: Facts and fallacies. Br Dent J. 2005.
- Matis BA. Degradation of gel in tray whitening. Compend Contin Educ Dent. 2000; 28: S28.
- Ferrari M, Kugel G, Cagidiaco MC, Barker ML, Gerlach RW. Clinical trial evaluating the peroxide concentration response of whitening strips over 28 days. Am J Dent. 2004; 17.
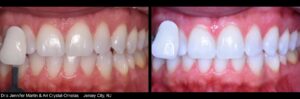
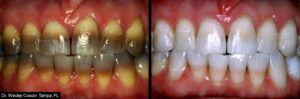
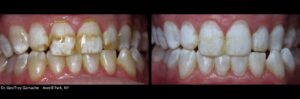
KöR® Whitening Gallery
Almost any whitening company can show you a few impressive before and after photos. But we have literally hundreds of cases, submitted by doctors like you, showing the phenomenal results you can get with the KöR® Whitening System.