At KöR Whitening, we often talk about how KöR is the only whitening system out there with a system that is truly based on science. And people give us those sideways, skeptical looks. How can that be? How can KöR be the only whitening system based entirely on science? That doesn’t make sense!
I believe I’ve been involved in teeth whitening research longer than anyone else on the planet. My first involvement in a tooth whitening study was in 1977 at Fairleigh Dickinson University.
Let’s talk about how current day teeth whitening came about. The story has it that a few decades ago, dentists in a study club were discussing how to try to make irritated and swollen gum tissue heal more quickly after removing orthodontic braces on teenage patients.
Normally, teenagers don’t do such a great job of brushing and flossing when they have braces. So when the braces are finally removed, the gums are inflamed and swollen.
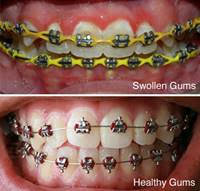
Some of the dentists in the study group brought up the topic of an over-the-counter product called Gly-Oxide®, which is still sold today.

Gly-Oxide is an oral antiseptic intended to help mouth sores heal more quickly. Some of the dentists suggested making “trays” that cover the teeth and gums of the patient. Gly-Oxide could be placed in the trays, and the trays worn over the teeth for a period of time every day. The dentists felt this may help the post-orthodontic gum inflammation and swelling heal more quickly.
But what these dentists noticed was that the teeth of these teenage patients seemed to become lighter in color. What’s up with that? Who knew?
These dentists all agreed that, for some unknown reason, use of Gly-Oxide in custom fitted trays over the teeth resulted in some whitening of the teeth. Well, as it turned out, Gly-Oxide’s active ingredient is 10% carbamide peroxide, which is the most common ingredient in teeth whitening gel used by patients at home even today.
So of course, corporate America jumped into the mix and provided products with 10% carbamide peroxide, calling them “teeth bleaching gels”. This was the start of teeth whitening as we know it today.
But we Americans are impatient people. We want results NOW! We don’t want to have to wear trays every day for 10 or more weeks simply to get our teeth a little whiter. So corporate America started making stronger whitening gel (stronger than 10% carbamide peroxide). That helped, but not enough.
As gels were made even stronger, it was found that stronger gels burned the gums. So “In-Office Power Whitening” was born. In the dental office, the gums can be protected by using something called rubber dam, and later something called “paint-on dam”, which is a protective coating painted over the soft gum tissue.
But this In-Office Power Whitening often didn’t work very well, and even when it did, it faded away after just a few days. So corporate America asked themselves how to make the breakdown of high concentration peroxide more effective.
Well, remember back in science class, you put a couple things in a little ceramic bowl and mix them. And nothing happens. But when you put that bowl over a Bunsen Burner flame to heat it up, the heat (energy) causes the chemical reaction to proceed.
So they decided to give heating of the peroxide a try. In fact, they used heating lamps shining on the teeth as they were being whitened with high concentration peroxide.
This caused so much heat that it damaged the nerves of teeth. Oops! Back to the drawing board.
So, instead of heat energy, corporate America decided to force energy by using high-intensity bleaching lights and lasers. So the idea was to shine photon (light and laser) energy onto the teeth to make the chemical reaction of the peroxide on the teeth go faster.
It certainly sounded good, and many patients and dentists alike “fell for it”, but the fact is that it didn’t work. And patients complained that their teeth didn’t get white, and even when they did get whiter, they were back to normal within a few days. Some patients even asked their dentists for a refund of their money.
Well, we dentists are pretty smart. We already knew that whitening at home using custom-made whitening trays works. So we started making whitening trays for the patients. After their In-Office Power Bleaching (often using a bleaching light or laser), dentists gave their patients the whitening trays and at-home whitening gel and instructed the patient to start wearing these trays right away.
And that’s pretty much where all whitening companies are even today…except for KöR Whitening.
As you can see, this had nothing to do with science. It was simply hit‘n miss, trial and error.
Let me give you just one example of how most present-day teeth whitening procedures are not based on science whatsoever: I discussed above how corporate America brought out bleaching lights and bleaching lasers to add photon energy into the peroxide breakdown reaction to make it work better/faster. That all sounds good, and it might even have worked… IF…the breakdown of hydrogen peroxide was an “endothermic” chemical reaction. But it’s NOT. The breakdown of hydrogen peroxide is an “exothermic” chemical reaction. And there’s a BIG difference between the two.
Endothermic chemical reactions REQUIRE energy (heat, light, electricity, etc.) added to the reaction to make it work. A great example is cooking an egg. The heat causes the proteins of the egg to go through a chemical process called denaturing, turning the runny, clear portion of the egg firm and white.
Instead of requiring energy for a reaction to proceed like an endothermic reaction, an exothermic reaction requires GETTING RID of energy (heat, light, etc.). A good example is the little plastic pack you place inside your gloves when you are in a cold, snowy environment. Inside the little plastic baggie are two different components. You squeeze the plastic baggie until the components inside the baggie pop and release their chemicals. Then you squish the baggie with your hands to mix the components inside. And what happens? The baggie gets warm.
The components inside the baggie go through an exothermic reaction, giving off heat energy. THAT is an exothermic reaction.
In fact, according to Le Chatelier’s Principle of Chemical Equilibrium, if you try to force energy (heat, light, etc.) into an exothermic reaction (which is trying to get rid of energy), you can actually slow the reaction down.
So where is the science behind using bleaching lights and lasers? There is none.
Through my teeth whitening research over the past 41 years, since 1977, I have learned and discovered the full science behind teeth whitening. What I’ve found is that all whitening companies out there have it backward. Of course, they do. Their procedures are based simply on throwing things against the wall to see what sticks (and sells). Today’s typical teeth whitening procedures do not follow what the sciences of chemistry, physics, physiology, and microanatomy dictate to get teeth truly and permanently white.
At KöR Whitening, I have developed a whitening system that truly follows what science dictates to achieve the amazing and permanent results that so many have already seen and become familiar with.
To learn more about the science of whitening and how KöR puts science to work, click here to download the free Science Paper.



Recent Comments